Protocol - Disease Progression and Regression - 0-24 Months
- Growth Charts
- Height - Knee Height
- Height - Recumbent Length
- Height - Self-Reported Height
- Height - Standing Height
- Weight - Measured Weight
- Weight - Self-Reported Weight
Description
The Newcastle Paediatric Mitochondrial Disease Scale (NPMDS) and the Newcastle Mitochondrial Disease Adult Scale (NMDAS) can be used to evaluate the progression of mitochondrial disease. There are three versions of the NPMDS, each for a specific age range (0-24 months, 2-11 years, and 12-18 years). The NMDAS is for adult patients over 16 years.
The scales allow for standardization of patient assessment and to improve accurate data collection. The scales are composed of multiple domains: Current Function, System Specific Involvement, Current Clinical Assessment and Quality of Life. Almost all questions provide a score which ranges from 0 to 3, with the following representations: 0 is normal, 1 is mild, 2 is moderate, and 3 is severe. Examples of severity for each question are provided. Depending upon the domain, the questions are either self- or interviewer-administrated, based on a provider’s clinical assessment or medical records. There is a manual for each scale, which details the administration, process, and scoring instructions.
Specific Instructions
In order to maximize consistency, the authors of the scale state it is essential that clinicians adhere to the scale instructions. They also advise that the scales be administrated by clinicians with experience in the care of patients with mitochondrial disease and also that the scale be given every 6 months for children under 2 years of age and at 6- to 12-month intervals for older children and adults.
Availability
This protocol is freely available; permission not required for use.
Protocol
The Newcastle Paediatric Mitochondrial Disease Scale (NPMDS)
0 - 24 months
Date of assessment:
Age at assessment:
Parental consanguinity:
Age at presentation:
Age at clinical diagnosis:
Clinical diagnosis:
Genotype if known:
Biochemical phenotype if known:
Basis of clinical diagnosis e.g. MRI, blood / CSF lactate
Information regarding pregnancy
• reduced fetal movements _____Y___ N___ unknown_____
• cardiomyopathy on antenatal scans ___ Y___ N___ unknown________
• abnormalities on fetal anomaly scan ___ Y___ N___unknown________ Please specify_______________________________________________
• other:
Neonatal information:
• gestational age _______weeks
• delivery method (NVD vs instrumental vs C/S) _______
• birth weight _______kg
• resuscitation and ventilation ___ Y___ N___unknown__ _______
Please specify_______________________________________________
Scores: Sections I-III:
Section IV:
Section I: Current Function
Rate function during the preceding 2 week period only according to caregiver
interview. Indicate the score that best fits patient’s functional status independently of the nature of the signs.
1. Vision
- Normal. No parental concerns
- Mild. Limited eye or head movement to large objects or parental face in visual field
- Moderate. No response to large objects or parental face in the visual field
- Severe. No response to light
2. Hearing
- Normal
- Mild. Body, head or eye movement only to loud noise
- Moderate. No reaction to loud noise
- Severe. No hearing (even with aid)
3. Communication (assessed with appropriate regard for age)
- Normal. Age appropriate communication
- Mild. Delayed development of communication
- Moderate. Communication unintelligible to parents or completely reliant on non-verbal communication
- Severe. Not communicating effectively in any form
4. Feeding
- Normal
- Mild. Difficulties in sucking / coughing / anorexia / wheezy with feeds or occasional choking
- Moderate. Supplementary enteral feeding or recurrent aspiration pneumonia
- Severe. Exclusive enteral feeding (gastrostomy / NG tube). Nil by mouth
5. Mobility
- Normal. No concerns. Age appropriate mobility
- Mild. Clumsy age appropriate mode of mobility
- Moderate. Mobile but through age inappropriate mode
- Severe. Immobile
Section II: System Specific Involvement
Rate system specific involvement during the preceding 6 month period only (or since birth if the child is less than 6 months old) unless otherwise stated in the question. Scores should be assigned according to caregiver interview, clinician’s knowledge of the patient and clinical notes.
1. Seizures
- None
- Mild. Myoclonic or absence seizures only or < 1 generalised tonic-clonic seizure/month
- Moderate. > 5 generalized tonic-clonic seizures/month or > 20 absence or myoclonic seizures/month
- Severe. Status epilepticus or intractable seizures
2. Encephalopathy
- None
- Mild. Abnormal sleepiness / lethargy. Waking only for feeds
- Moderate. Recurrent episodes of mild encephalopathy (> 2/year)
- Severe. Life threatening encephalopathy - requires artificial ventilation
3. Gastrointestinal
- Normal.
- Mild. Constipation or unexplained vomiting / diarrhoea > 3/week
- Moderate. Severe constipation (no relief with laxative treatment) or unexplained vomiting / diarrhoea every day or surgical intervention for dysmotility
- Severe Malabsorption / Failure to thrive
4. Endocrine
- Normal.
- Mild. Biochemical evidence of impaired function
- Moderate. Endocrine failure requiring replacement therapy
- Severe. Endocrine decompensation (e.g. diabetic ketoacidosis, Addisonian crisis)
5. Respiratory
- Normal
- Mild. Abnormal respiratory pattern not requiring therapy / hospitalization
- Moderate. Abnormal respiration requiring oxygen flow or hospitalisation but not ventilation
- Severe. Abnormal respiration requiring artificial ventilation
6. Cardiovascular- over preceding 12 months
- Normal
- Mild. Asymptomatic ECG change
- Moderate. Abnormal echocardiogram (e.g. cardiomegaly) or sustained / symptomatic arrhythmia on ECG
- Severe. Decompensated cardiomyopathy or requiring pacing device / defibrillator / ablation
7. Renal
- Normal
- Mild. Impaired function but no change in diet or therapy required
- Moderate. Impaired function requiring restricted protein diet
- Severe. Failure requiring transplant / dialysis
8. Liver
- Normal
- Mild. Mildly impaired Liver Function Tests (LFTs). Normal albumin and coagulation. No symptoms of hepatic failure
- Moderate. Impaired LFTs with symptoms (e.g. jaundice, coagulation anomalies, oedema)
- Severe. Failure requiring hospitalisation and / or transplantation
9. Blood
- Normal
- Mild. Anaemia only
- Moderate. Asymptomatic pancytopenia
- Severe. Pancytopenia requiring regular transfusion / transplantation
Section III: Current Clinical Assessment
Rate current status according to the clinician’s examination at the time of assessment unless otherwise stated in the question.
1. Growth (weight) over preceding 6 months
- Normal. Following normal growth trajectory
- Mild. Weight less than second centile but growing parallel to it
- Moderate. Weight crossing one centile
- Severe. Weight crossing ≥ 2 centiles or less than 2nd centile with divergent trajectory
2. Development over preceding 4 months Score: ____
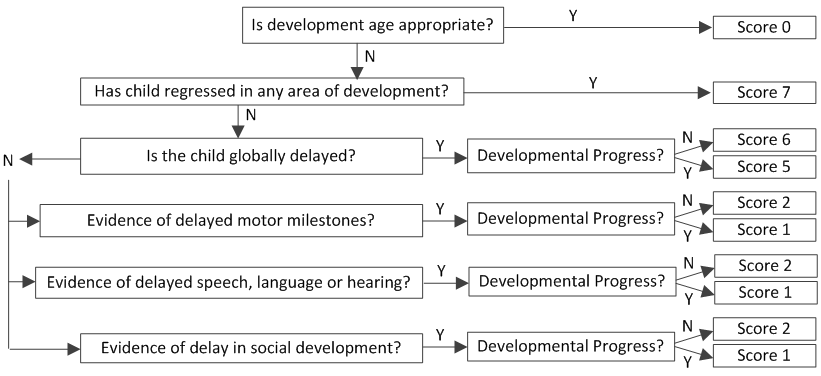
3. Vision
- Normal. Normal fixation and tracking
- Mild. Impaired fixation and / or tracking of small objects
- Moderate. Impaired fixation and / or tracking of familiar faces
- Severe. No response to light or registered blind
4. Ptosis and Eye Movement
- Normal
- Mild. Gaze evoked nystagmus or unilateral ptosis or impaired eye movement at extremities
- Moderate. Intermittent nystagmus at rest or bilateral ptosis not obscuring pupils or restriction of >50% eye movement
- Severe. Continuous nystagmus at rest or bilateral ptosis obscuring pupils or only a flicker of eye movement
5. Myopathy
- Normal
- Mild. Mild symmetrical weakness of hip and / or shoulder girdle only
- Moderate. Moderate symmetrical weakness (proximal > distal) limiting functional movement
- Severe. Wheelchair / carrier dependent or respiratory compromise due to myopathy.
6. Pyramidal
- Normal
- Mild. Unilateral pyramidal signs but retaining functional movement
- Moderate. Dense hemiplegia with little movement of affected side
- Severe. Bilateral pyramidal weakness with little or no movement
7. Extrapyramidal
- Normal.
- Mild. Unilateral extrapyramidal posturing and increased tone
- Moderate. Bilateral extrapyramidal posturing and increased tone
- Severe. Severe extrapyramidal posturing resulting in very little movement
8. Neuropathy
- Normal.
- Mild. Areflexia only
- Moderate. Sensory ataxia or motor impairment (distal weakness) but mobile
- Severe. Reliant on mobility aids primarily due to neuropathy
Section IV: Quality of Life
This survey asks for your views about your child’s recent health. Please answer every question by marking an ‘x’ in the box next to the phrase which best describes your answer.
1) During the past 4 weeks, how would you rate your child’s overall health?
[ ] Very poor
[ ] Poor
[ ] Fair
[ ] Good
[ ] Very good
2) During the past 4 weeks, how much did your child’s physical health problems limit their physical activities (such as moving or playing)?
[ ] Very much
[ ] Quite a lot
[ ] Somewhat
[ ] A little
[ ] Not at all
3) During the past 4 weeks, how much energy did your child have?
[ ] None
[ ] A little
[ ] Some
[ ] Quite a lot
[ ] Very much
4) During the past 4 weeks, how much bodily pain / discomfort did your child have?
[ ] Very much
[ ] Quite a lot
[ ] Some
[ ] A little
[ ] None
5) During the past 4 weeks, how would you rate your child’s behaviour compared with other children his / her age?
[ ] Very poor
[ ] Poor
[ ] Fair
[ ] Good
[ ] Very good
6) During the past 4 weeks, how would you rate your child’s ability to interact with other people (e.g. with you, siblings or other children his / her age) compared with other children his / her age?
[ ] Very poor
[ ] Poor
[ ] Fair
[ ] Good
[ ] Very good
7) During the past 4 weeks, how much were you (the parent / carer) bothered by emotional problems (e.g. feelings of anxiety, sadness) as a result of your child’s illness?
[ ] Very
[ ] Quite a lot
[ ] Somewhat
[ ] A little
[ ] Not at all
8) During the past 4 weeks, how much was your time limited as a result of your child’s illness?
[ ] Very
[ ] Quite a lot
[ ] Somewhat
[ ] A little
[ ] Not at all
9) During the past 4 weeks, how much were your family’s activities limited or interrupted as a result of your child’s illness?
[ ] Very
[ ] Quite a lot
[ ] Somewhat
[ ] A little
[ ] Not at all
10) During the past 6 months, what has been the financial cost of your child’s illness?
[ ] Very expensive
[ ] Quite expensive
[ ] Moderately expensive
[ ] Little additional cost
[ ] No additional cost
11) During the past 4 weeks, how would you rate your family’s ability to get along with one another?
[ ] Very poor
[ ] Poor
[ ] Fair
[ ] Good
[ ] Very good
12) During the past 4 weeks, how often did your child’s illness have a positive effect on your child, you or your family (e.g. being treated well due to illness, meeting new people)?
[ ] Never
[ ] Occasionally
[ ] Sometimes
[ ] Quite a lot
[ ] Most of the time
Personnel and Training Required
The Newcastle Paediatric Mitochondrial Disease Scale (NPMDS) and the Newcastle Mitochondrial Disease Adult Scale (NMDAS) should be administered by clinicians, preferably with experience in caring for patients with mitochondrial disease or other rare genetic conditions.
Equipment Needs
None.
Requirements
| Requirement Category | Required |
|---|---|
| Major equipment | No |
| Specialized training | Yes |
| Specialized requirements for biospecimen collection | No |
| Average time of greater than 15 minutes in an unaffected individual | Yes |
Mode of Administration
Proxy-administered questionnaire
Lifestage
Infant, Toddler
Participants
Children ages 0-24 months
Selection Rationale
The Rare Genetic Conditions Working Group (WG) selected the Newcastle Paediatric Mitochondrial Disease Scale (NPMDS) and the Newcastle Mitochondrial Disease Adult Scale (NMDAS) because mitochondrial disorders have a wide array of symptoms making this measure potentially extensible to other progressive disorders. In addition, rare genetic disorders can be associated with secondary mitochondrial dysfunction and overlapping symptoms. Although these scales were developed for mitochondrial diseases, the WG acknowledges data from these scales can be beneficial for other rare genetic conditions, such as inborn errors of metabolism, storage disorders, and nonmitochondrial myopathies.
Language
English
Standards
| Standard | Name | ID | Source |
|---|---|---|---|
| Human Phenotype Ontology | Pace of progression | HP:0003679 | HPO |
| caDSR Form | PhenX PX220701 - Disease Progression And Regression 0-24 Months | 6201956 | caDSR Form |
Derived Variables
None
Process and Review
Not applicable.
Protocol Name from Source
Newcastle Paediatric Mitochondrial Disease Scale (NPMDS), 0-24 months
Source
Newcastle University. Newcastle Paediatric Mitochondrial Disease Scale (NPMDS)
0-24 months.
Available at bsu.ncl.ac.uk/pdfs/0-2_paed.pdf.
General References
Enns, G. M., Moore, T., Le, A., Atkuri, K., Shah, M. K., Cusmano-Ozog, K., Niemi, A. K., & Cowan, T. M. (2014). Degree of glutathione deficiency and redox imbalance depend on subtype of mitochondrial disease and clinical status. PLoS One, 9(6), e100001. doi:10.1371/journal.pone.0100001
Phoenix, C., Schaefer, A. M., Elson, J. L., Morava, E., Bugiani, M., Uziel, G., Smeitink, J. A., Turnbull, D. M., & McFarland, R. (2006). A scale to monitor progression and treatment of mitochondrial disease in children. Neuromuscular disorders: NMD, 16(12), 814-820.
Protocol ID
220701
Variables
Export Variables| Variable Name | Variable ID | Variable Description | dbGaP Mapping | |
|---|---|---|---|---|
| PX220701_Disease_ProgressionRegression_024Mo_Abnormalities_FetalScan | ||||
| PX220701110000 | abnormalities on fetal anomaly scan | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_Abnormalities_Specify | ||||
| PX220701120000 | Please specify | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_Age | ||||
| PX220701020000 | Age at assessment: | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_Basis_Diagnosis | ||||
| PX220701080000 | Basis of clinical diagnosis e.g. MRI, blood more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_Biochemical_Phenotype | ||||
| PX220701070000 | Biochemical phenotype if known: | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_CurrentClinical_Development | ||||
| PX220701360000 | Rate current status according to the more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_CurrentClinical_Extrapyramidal | ||||
| PX220701410000 | Rate current status according to the more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_CurrentClinical_GrowthWeight | ||||
| PX220701350000 | Rate current status according to the more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_CurrentClinical_Myopathy | ||||
| PX220701390000 | Rate current status according to the more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_CurrentClinical_Neuropathy | ||||
| PX220701420000 | Rate current status according to the more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_CurrentClinical_PtosisEyeMovement | ||||
| PX220701380000 | Rate current status according to the more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_CurrentClinical_Pyramidal | ||||
| PX220701400000 | Rate current status according to the more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_CurrentClinical_Vision | ||||
| PX220701370000 | Rate current status according to the more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_CurrentFunction_Communication | ||||
| PX220701230000 | Rate function during the preceding 2 week more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_CurrentFunction_Feeding | ||||
| PX220701240000 | Rate function during the preceding 2 week more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_CurrentFunction_Hearing | ||||
| PX220701220000 | Rate function during the preceding 2 week more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_CurrentFunction_Mobility | ||||
| PX220701250000 | Rate function during the preceding 2 week more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_CurrentFunction_Vision | ||||
| PX220701210000 | Rate function during the preceding 2 week more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_Date | ||||
| PX220701010000 | Date of assessment: | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_Genotype | ||||
| PX220701060000 | Genotype if known: | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_Neonatal_BirthWeight | ||||
| PX220701160000 | birth weight in kg | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_Neonatal_DeliveryMethod | ||||
| PX220701150000 | delivery method (NVD vs instrumental vs C/S) | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_Neonatal_GestationalAge | ||||
| PX220701140000 | gestational age ____weeks | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_Neonatal_ResuscitationVentilation | ||||
| PX220701170000 | Resuscitation and ventilation | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_Neonatal_Specify | ||||
| PX220701180000 | Specify | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_ParentalAge_AgeDx | ||||
| PX220701050000 | Age at clinical diagnosis: | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_Parental_AgePresentation | ||||
| PX220701040000 | Age at presentation: | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_Parental_Consanguinity | ||||
| PX220701030000 | Parental consanguinity: | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_Pregnancy_Cardiomyopathy_Antenatal | ||||
| PX220701100000 | cardiomyopathy on antenatal scans | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_Pregnancy_Cardiomyopathy_PregnancyOther | ||||
| PX220701130000 | Other | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_QOL_ActivitiesLimited | ||||
| PX220701510000 | During the past 4 weeks, how much were your more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_QOL_Behavior | ||||
| PX220701470000 | During the past 4 weeks, how would you rate more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_QOL_EmotionalProblems | ||||
| PX220701490000 | During the past 4 weeks, how much were you more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_QOL_Energy | ||||
| PX220701450000 | During the past 4 weeks, how much energy did more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_QOL_FinancialCost | ||||
| PX220701520000 | During the past 6 months, what has been the more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_QOL_GetAlong | ||||
| PX220701530000 | During the past 4 weeks, how would you rate more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_QOL_Interactions | ||||
| PX220701480000 | During the past 4 weeks, how would you rate more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_QOL_OverallHealth | ||||
| PX220701430000 | During the past 4 weeks, how would you rate more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_QOL_Pain | ||||
| PX220701460000 | During the past 4 weeks, how much bodily more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_QOL_PhsyicalActivities | ||||
| PX220701440000 | During the past 4 weeks, how much did your more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_QOL_PositiveEffects | ||||
| PX220701540000 | During the past 4 weeks, how often did your more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_QOL_TimeLimited | ||||
| PX220701500000 | During the past 4 weeks, how much was your more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_Reduced_FetalMovement | ||||
| PX220701090000 | reduced fetal movements | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_Score_SectionIV | ||||
| PX220701200000 | Score Section IV: | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_Score_SectionsI_III | ||||
| PX220701190000 | Score Sections I-III | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_SystemInvolvement_Blood | ||||
| PX220701340000 | Rate system specific involvement during the more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_SystemInvolvement_Cardiovascular | ||||
| PX220701310000 | Rate system specific involvement during the more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_SystemInvolvement_Encephalopathy | ||||
| PX220701270000 | Rate system specific involvement during the more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_SystemInvolvement_Endocrine | ||||
| PX220701290000 | Rate system specific involvement during the more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_SystemInvolvement_Gastrointestinal | ||||
| PX220701280000 | Rate system specific involvement during the more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_SystemInvolvement_Liver | ||||
| PX220701330000 | Rate system specific involvement during the more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_SystemInvolvement_Renal | ||||
| PX220701320000 | Rate system specific involvement during the more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_SystemInvolvement_Respiratory | ||||
| PX220701300000 | Rate system specific involvement during the more | N/A | ||
| PX220701_Disease_ProgressionRegression_024Mo_SystemInvolvement_Seizures | ||||
| PX220701260000 | Rate system specific involvement during the more | N/A | ||
Measure Name
Disease Progression and Regression
Release Date
April 30, 2015
Definition
This measure determines the impact of disease on an individual over time.
Purpose
This measure is used to assess the presence and degree of symptoms over time. Rare genetic diseases, such as mitochondrial disorders and mucopolysaccharidosis (MPS), can be associated with symptoms that become more severe over time and may result in a regression of some physical abilities. This measure can be used to quantify such changes to determine the natural course of disease(s) as well as contribute to longitudinal or therapeutic intervention studies.
Keywords
Newcastle Paediatric Mitochondrial Disease Scale, NPMDS, Newcastle Mitochondrial Disease Adult Scale, NMDAS, mitochondria, Developmental Delay, Intellectual Delay, disease, disease scale, progression, regression
Measure Protocols
| Protocol ID | Protocol Name |
|---|---|
| 220701 | Disease Progression and Regression - 0-24 Months |
| 220702 | Disease Progression and Regression - 2-11 Years |
| 220703 | Disease Progression and Regression - 12-18 Years |
| 220704 | Disease Progression and Regression - Adult |