Protocol - Pain Location Body Map - Child
Description
The Childhood Arthritis and Rheumatology Research Alliance (CARRA) Pain Chart is a self-administered body map with 21 scored areas that can be completed either in paper or electronic format. Participants color or click on the location(s) that they have felt pain during the preceding 2 weeks. This protocol was validated in individuals up to 18 years of age.
Specific Instructions
Within von Baeyer et al. (2011), it is recommended that the pain chart be used without an adults assistance for children aged 7 years and older. The pain chart may be able to be completed by children younger than 7 years with adult assistance, but the adults influence must be recognized.
Availability
This protocol is freely available; permission not required for use.
Protocol
CARRA Pain Chart
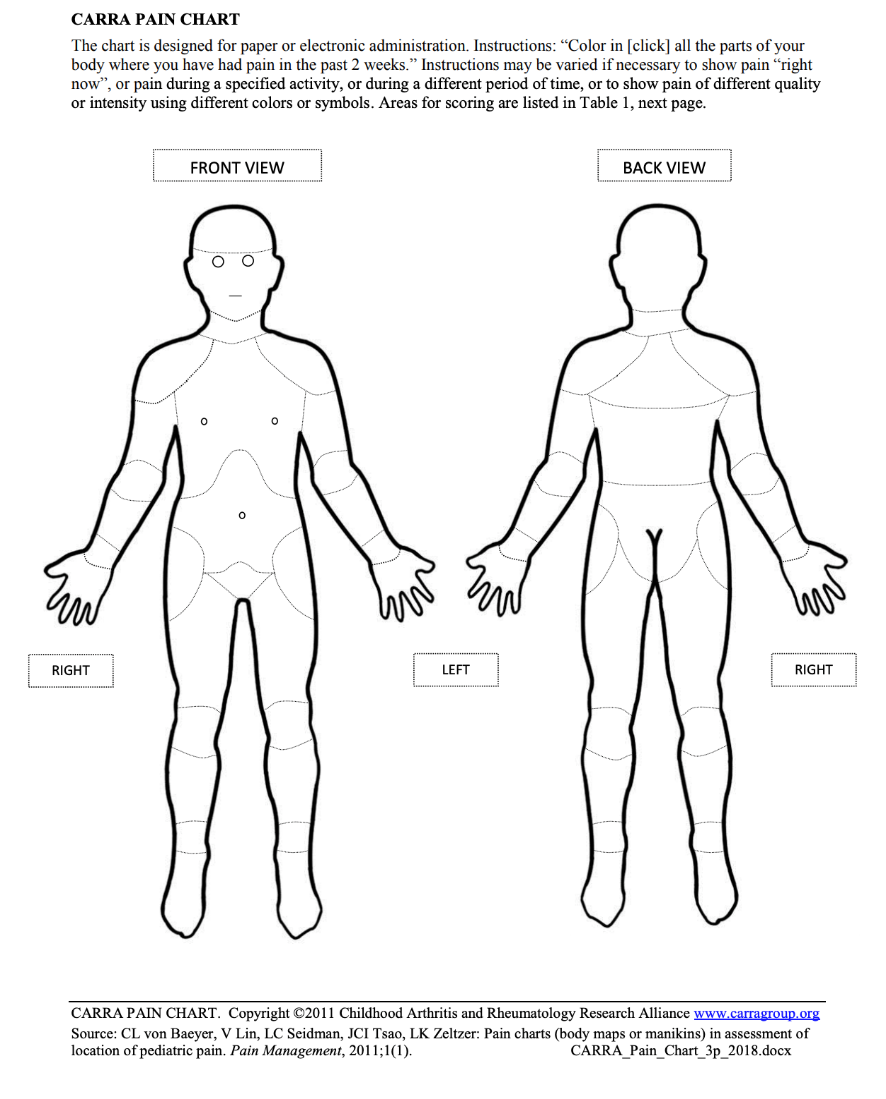
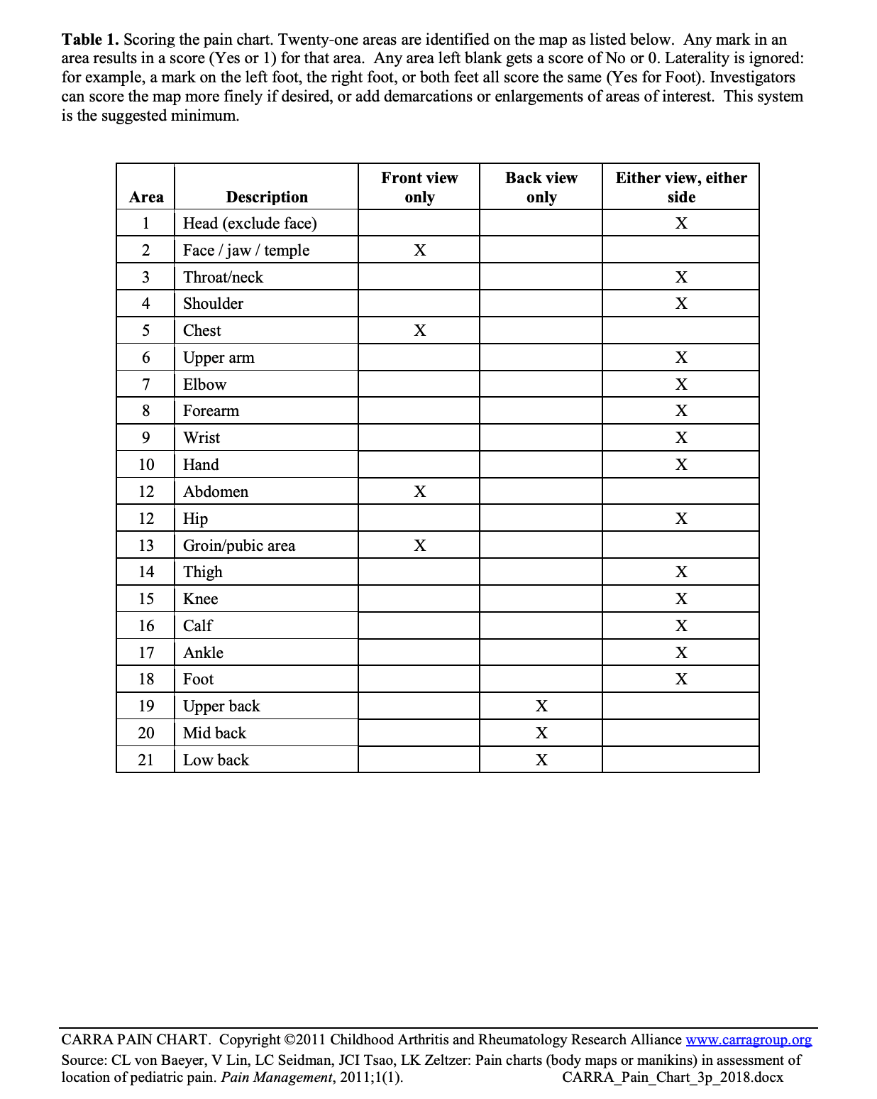
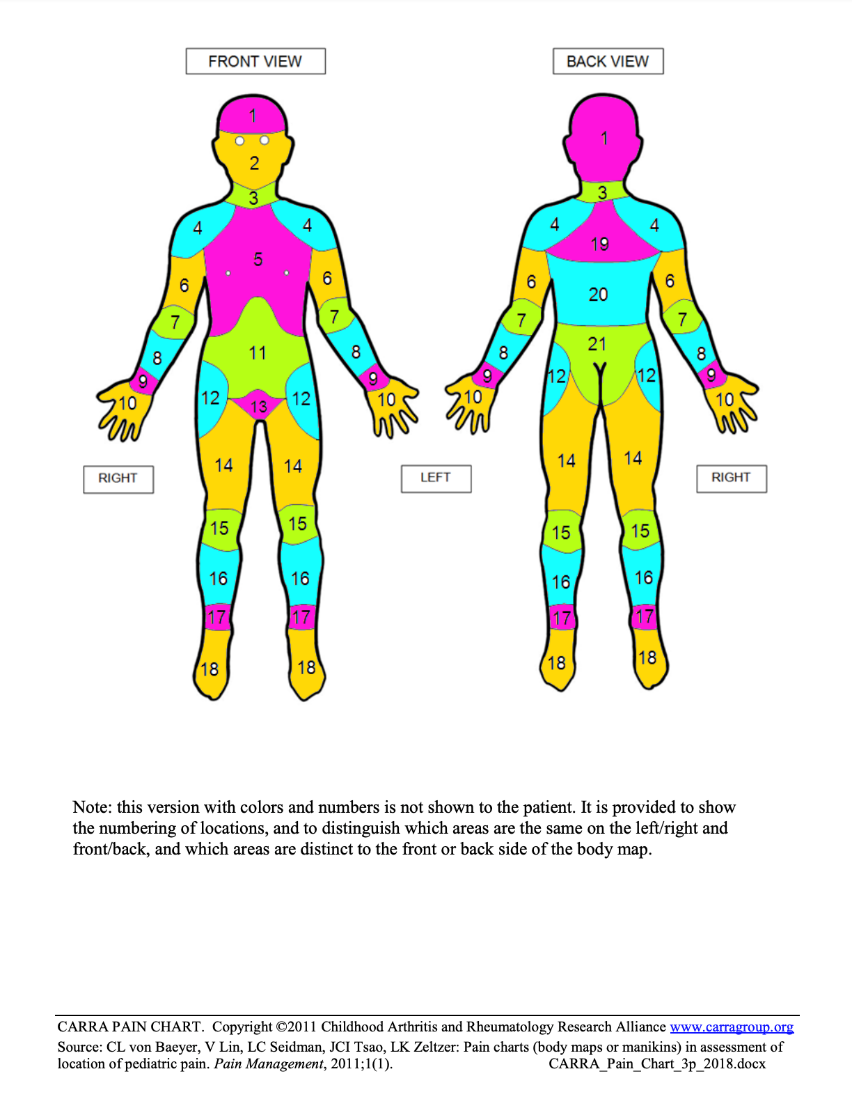
Personnel and Training Required
None
Equipment Needs
None
Requirements
| Requirement Category | Required |
|---|---|
| Major equipment | No |
| Specialized training | No |
| Specialized requirements for biospecimen collection | No |
| Average time of greater than 15 minutes in an unaffected individual | No |
Mode of Administration
Self-administered questionnaire
Lifestage
Child, Adolescent
Participants
Children and Adolescents up to 18 years of age
Selection Rationale
The Childhood Arthritis and Rheumatology Research Alliance (CARRA) Pain Chart is a single dimensional instrument that can be used across a wide age range (4-18 years) and within a short completion time
Language
English
Standards
| Standard | Name | ID | Source |
|---|---|---|---|
| caDSR Form | Px860602 Px860602 | 14861078 | caDSR Form |
Derived Variables
None
Process and Review
Not Applicable
Protocol Name from Source
Childhood Arthritis and Rheumatology Research Alliance (CARRA) Pain Chart
Source
von Baeyer, C. L., Lin, V., Seidman, L. C., Tsao, J. C., & Zeltzer, L. K. (2011). Pain charts (body maps or manikins) in assessment of the location of pediatric pain. Pain Management, 1(1), 61-68.
General References
Evans, S., Djilas, V., Seidman, L. C., Zeltzer, L. K., & Tsao, J. (2017). Sleep quality, affect, pain, and disability in children with chronic pain: Is affect a mediator or moderator? Journal of Pain, 18(9), 1087-1095.
Luca, N. J., Stinson, J. N., Feldman, B. M., Benseler, S. M., Beaton, D., Campillo, S., LeBlanc, C., van Wyk, M., & Bayoumi, A. M. (2017). Validation of the Standardized Universal Pain Evaluations for Rheumatology Providers for Children and Youth (SUPER-KIDZ). Journal of Orthopaedic & Sports Physical Therapy, 47(10), 731-740.
Stinson, J. N., Connelly, M., Jibb, L. A., Schanberg, L. E., Walco, G., Spiegel, L. R., Tse, S. M., Chalom, E. C., Chira, P., & Rapoff, M. (2012). Developing a standardized approach to the assessment of pain in children and youth presenting to pediatric rheumatology providers: A Delphi survey and consensus conference process followed by feasibility testing. Pediatric Rheumatology Online Journal, 10(1), 7.
Protocol ID
860602
Variables
Export Variables| Variable Name | Variable ID | Variable Description | dbGaP Mapping | |
|---|---|---|---|---|
| PX860602_Sickle_Cell_Pain_Location_Body | ||||
| PX860602010000 | Select all parts of your body where you have more | N/A | ||
Measure Name
Pain Location
Release Date
May 18, 2022
Definition
This measure assesses the number and location of sites on the body where pain symptoms are felt.
Purpose
The number and location of pain sites vary significantly by age, frequency of pain, crisis, and utilization. Pain location can predict important behavioral outcomes, number of days where an individual experiences subjective vaso-occlusive crises, and unplanned utilization of hospitals.
Keywords
pain, response to pain, sickle cell disease, SCD, location, site of pain, Sickle Cell Disease Pain Diary, PiSCES project
Measure Protocols
| Protocol ID | Protocol Name |
|---|---|
| 860601 | Sickle Cell Disease Pain Location Body Map - Adult |
| 860602 | Pain Location Body Map - Child |